Introduction: Advanced systemic mastocytosis (AdvSM) comprises a heterogeneous group of clonal mast cell neoplasms, primarily driven by KIT D816V. Measures of AdvSM response, including the International Working-Group for Myeloproliferative Neoplasms Research and Treatment and European Competence Network on Mastocytosis (IWG-MRT-ECNM) criteria, are based on improvements in mast cell-related organ damage (C-findings), and further sub-classified by the extent of reduction in measures of mast cell disease (e.g. serum tryptase level, bone marrow mast cell burden). However, assessment of some C-findings lacks precision (such as splenomegaly and its resolution). Normalization of C-findings may not be an adequate surrogate for important clinical outcomes such as overall survival (OS). We evaluated whether pure pathologic response (PPR) criteria based on changes in bone marrow mast cells, serum tryptase, and complete blood count was more closely correlated with OS compared to the modified IWG-MRT-ECNM (mIWG-MRT-ECNM) criteria.
Methods: As an exploratory post-hoc analysis of the phase 1 EXPLORER study of avapritinib in AdvSM, we evaluated responses lasting ≥12 weeks by both mIWG-MRT-ECNM and PPR criteria. At baseline, evaluability for mIWG-MRT-ECNM response required ≥1 evaluable C-findings; PPR required presence of bone marrow mast cell aggregates and/or serum tryptase ≥20 ng/mL. Per PPR, morphologic complete remission (mCR) is absence of bone marrow mast cell aggregates, serum tryptase <20 ng/mL and full (or partial [mCRh]) hematologic recovery; morphologic partial remission (mPR) is ≥50% reduction in bone marrow mast cells and serum tryptase level. OS was analyzed by Kaplan-Meier method and was time from first dose to death. OS comparisons were by log-rank test, performed for best response and landmark analyses at various cycles.
Results: As of the data cut-off of August 30, 2019, 80 patients enrolled including 62 with AdvSM (7 with aggressive SM [ASM], 44 SM with an associated hematologic neoplasm [SM-AHN] and 11 with mast cell leukemia [MCL]). Ten (16%) AdvSM patients (7 ASM, 3 SM-AHN) were not response evaluable (RE) per mIWG-MRT-ECNM criteria, due to a lack of an evaluable C-finding at baseline, and 4 additional AdvSM patients were recently enrolled and were not yet response evaluable.
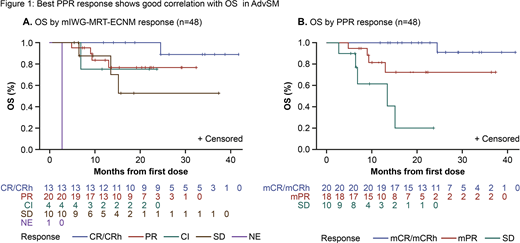
Of the 48 RE patients (3 ASM, 35 SM-AHN and 10 MCL) the best overall response rate (ORR) per mIWG-MRT-ECNM was 77% (8% CR, 19% CRh, 42% partial response [PR], and 8% clinical improvement [CI]). Non-responders had stable disease (SD; 21%) or were not evaluable (NE) due to insufficient (<13 weeks) follow-up (2%). Responders (CR/CRh/PR/CI) had 18-month OS of 85% (CR/CRh, 100%; PR, 77%; CI, 75%; Figure 1A); non-responders had 18-month OS of 48% (SD, 53%; NE, 0%) (P=0.042). Per PPR criteria, the best ORR was similar at 79%; however, a greater proportion of patients were assessed as being in a complete remission (15% mCR, 27% mCRh and 38% mPR). Non-responders by PPR all had SD (21%). This demonstrates that elimination of measurable mast cell burden can be discordant with complete C-finding resolution. Responders (mCR/mCRh/mPR) by PPR had 18-month OS of 88% (mCR/mCRh: 100%; mPR: 72%; Figure 1B); non-responders (all SD) had 18-month OS of 21% (P=0.0001).
Eventually, all 62 AdvSM patients will be evaluable by PPR criteria, including those 10 patients without mIWG evaluable C-findings at baseline; however, 5 patients had insufficient follow-up at the time of analysis. For the 57 AdvSM patients with sufficient follow up, the best ORR per PPR criteria was similar at 77% (14% mCR, 26% mCRh and 37% mPR). Overall, no patients had a best response of progressive disease based on mIWG or PPR criteria.
Landmark analyses of PPR at the end of 6 cycles showed a trend in 18-month OS of mCR/mCRh>mPR>SD in patients with similar starting avapritinib doses of ≥200 mg daily (n=48 of 57 PPR-evaluable patients).
Conclusions: In the phase I EXPLORER study, response assessment in AdvSM using PPR criteria increases the evaluable population, significantly correlates with OS, and should be explored as a potential primary endpoint for future trials.
Gotlib:Blueprint Medicines Corporation: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Chair of the Response Adjudication Committee and Research Funding, Research Funding; Deciphera: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: co-chair of the Study Steering Committee and Research Funding. Radia:Blueprint Medicines Corporation: Membership on an entity's Board of Directors or advisory committees; Novartis: Membership on an entity's Board of Directors or advisory committees, Other: Education events. George:Blueprint Medicines Corporation: Consultancy, Other: I have received no funding for this research. ARUP Laboratories, owned by the University of Utah, has received funding; Allakos: Consultancy; Deciphera: Other: consultancy, but has received no financial compensation for the past 12 months; Celgene: Consultancy. Robinson:Blueprint Medicines Corporation: Research Funding. Drummond:Jazz: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Pfizer: Consultancy, Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Gilead: Membership on an entity's Board of Directors or advisory committees; Bristol Myers Squibb: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Takeda: Membership on an entity's Board of Directors or advisory committees; Astellas: Membership on an entity's Board of Directors or advisory committees; Blueprint Medicine Corporation: Research Funding. Bose:Incyte Corporation: Consultancy, Honoraria, Research Funding, Speakers Bureau; Kartos Therapeutics: Honoraria, Research Funding; Astellas Pharmaceuticals: Research Funding; NS Pharma: Research Funding; Pfizer, Inc.: Research Funding; Promedior, Inc.: Research Funding; Constellation Pharmaceuticals: Research Funding; Celgene Corporation: Honoraria, Research Funding; CTI BioPharma: Honoraria, Research Funding; Blueprint Medicines Corporation: Honoraria, Research Funding. Hexner:Samus Therapeutics: Research Funding; Novartis: Research Funding; American Board of Internal Medicine: Other: member of the hematology exam committee; Blueprint Medicines Corporation: Other: serves on a data safety monitoring committee, Research Funding. Winton:Blueprint Medicines Corporation: Research Funding; Samus Therapeutics: Research Funding; Incyte Corporation: Research Funding. Horny:Novartis: Consultancy; Blueprint Medicines Corporation: Consultancy. Tugnait:Blueprint Medicines Corporation: Current equity holder in publicly-traded company, Ended employment in the past 24 months. Schmidt-Kittler:Blueprint Medicines Corporation: Current equity holder in publicly-traded company, Ended employment in the past 24 months. Evans:Blueprint Medicines Corporation: Current equity holder in publicly-traded company, Ended employment in the past 24 months. Lin:Blueprint Medicines Corporation: Current Employment, Current equity holder in publicly-traded company. Mar:Blueprint Medicines Corporation: Current Employment, Current equity holder in publicly-traded company. Deininger:Leukemia & Lymphoma Society: Research Funding; Bristol-Myers Squibb: Consultancy, Honoraria, Other, Research Funding; Ariad: Consultancy, Honoraria, Other; Incyte: Consultancy, Honoraria, Other, Research Funding; Novartis: Consultancy, Other, Research Funding; Medscape: Consultancy; Takeda: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Fusion Pharma: Consultancy; Blueprint Medicines Corporation: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: part of a study management committee, Research Funding; DisperSol: Consultancy; Galena: Consultancy, Honoraria, Other; Celgene: Research Funding; Gilead Sciences: Research Funding; SPARC: Research Funding; Sangamo: Consultancy, Membership on an entity's Board of Directors or advisory committees; Pfizer: Honoraria, Other, Research Funding. DeAngelo:Incyte Corporation: Consultancy; Glycomimetics: Research Funding; Forty-Seven: Consultancy; Amgen: Consultancy; Novartis: Consultancy, Research Funding; Pfizer: Consultancy; Abbvie: Research Funding; Jazz: Consultancy; Autolos: Consultancy; Shire: Consultancy; Takeda: Consultancy; Blueprint Medicines Corporation: Consultancy, Research Funding; Agios: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal